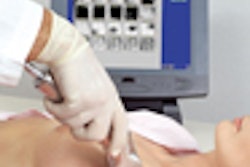
The Food and Drug Administration has granted its first-ever clearance for the soft-copy reading of breast images collected with a full-field digital mammography system. The agency granted the approval to the Senographe 2000D product from GE Medical Systems, the company has announced.
The approval comes less than 10 months after the agency's approval of Senographe 2000D for hard-copy image review. The FDA's action this month will help make digital mammography more accessible to women in the U.S., GE said. Waukesha, WI-based GE has the first, and so far the only, FDA-approved full-field digital mammography system on the U.S. market.
Senographe 2000D cuts breast exam time in half, decreases callbacks, and offers better visibility of the entire breast area than comparable film-based systems, according to GE. Because the image is displayed on the workstation just 10 seconds after exposure, the system offers a quick way to verify patient positioning.
The company has installed 35 Senographe 2000D systems in the U.S. to date, and said it expects the total to reach 60 before the end of 2000.
By Auntminnie.com staff writersNovember 17, 2000
Related Reading
First digital mammo system hooked up in western PA, September 14, 2000
GE partners with IFT for digital x-ray, June 28, 2000
GE to launch new digital x-ray systems, February 29, 2000
Digital mammography to face tough implementation hurdles, February 7, 2000
FDA approves GE's full-field digital mammography system, January 31, 2000
FDA panel votes to recommend GE digital mammo unit, December 17, 1999
FDA panel to review GE's digital mammography system, December 16, 1999
Copyright © 2000 AuntMinnie.com